Why is the world suffering from a penicillin shortage?
Once considered a miracle drug, few companies now make the antibiotic, and antibiotic resistance is rising as a result.

Stella Ngubenkomo was 11 years old when she found out that she suffered from a lifelong heart condition and would require monthly injections of penicillin to avoid heart failure and premature death.
“My joints used to be very painful and I would get tired easily. Then my mum took me to the hospital and we learned about my disease. The doctor said I wouldn’t live long,” she recalls.
Keep reading
list of 4 itemsWhat is behind the insulin shortage in the US?
Deaf Syrian boy hears for first time after life-changing operation
Aslan, a little Syrian boy’s journey to hear again
For the past 16 years, the resident of Cape Town, South Africa, has gone to a local clinic once a month to receive an injection of penicillin, the only drug that can prevent further damage to her heart valves and keep her alive. But, over the past two years, a shortage of the antibiotic has impacted the delivery of treatment to patients across South Africa.

“At the start of 2015 we experienced shortages of benzathine penicillin G. We then switched to using ampicillin, but then we ran out of ampicillin as well,” says Mark Sonderup, vice-chairman of the South African Medical Association (SAMA).
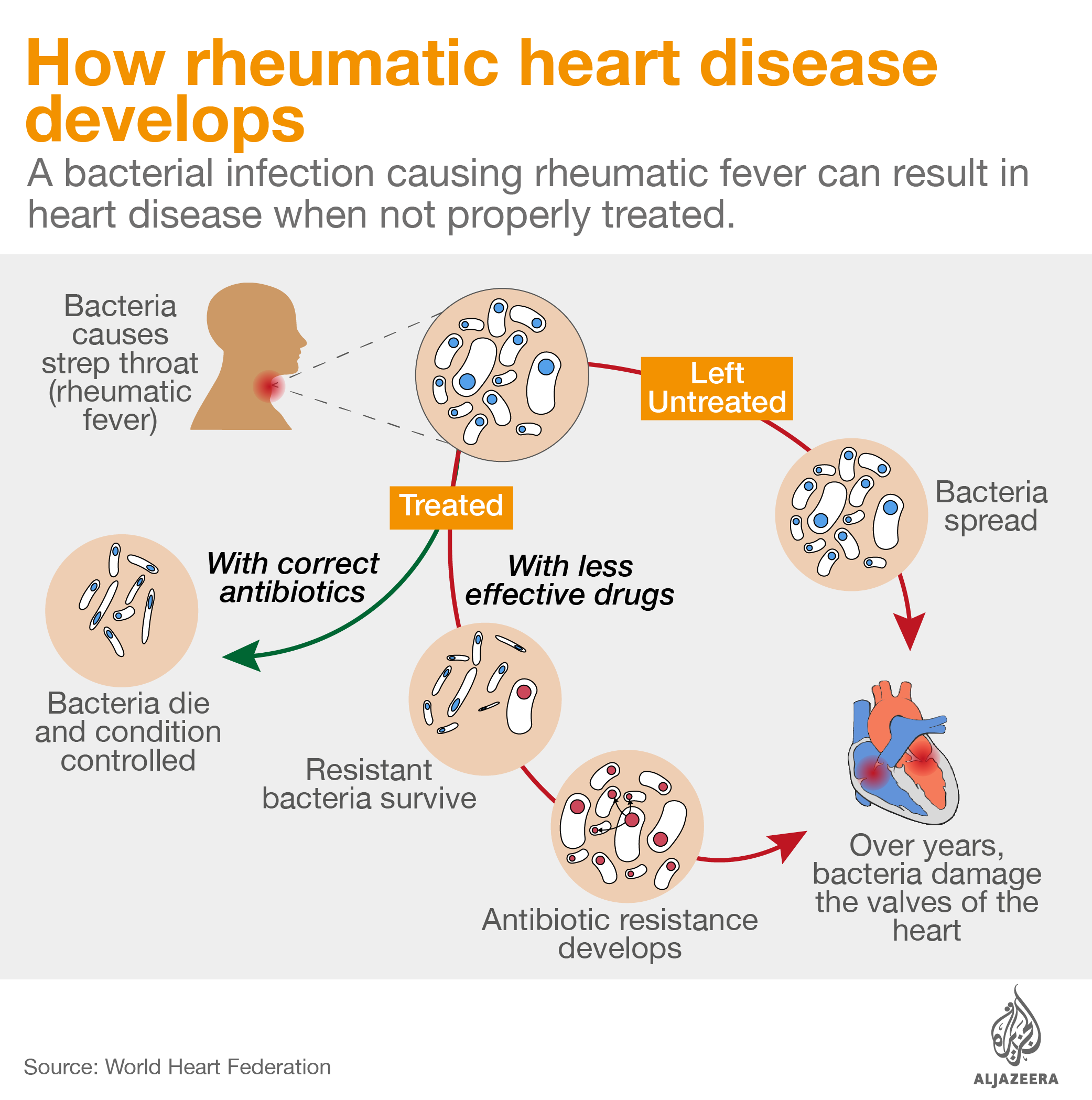
Intramuscular antibiotic benzathine penicillin G is critical to control rheumatic heart disease, an illness that kills thousands of people every year and from which Stella – and 33 million others worldwide – suffer. Patients can develop the illness from a simple strep throat infection. Strep throat can be easily cured with a single injection of penicillin, but if left untreated, the bacteria causing the infection can infect other parts of the body, including the heart, damaging the valves and changing the lives of patients forever.
![Stella Ngubenkomo at Groote Schuur Hospital in Cape Town, South Africa [Courtesy of Michael Walker]](/wp-content/uploads/2017/05/e6df5472439e444a8932c05ebbe7c7d1_18.jpeg)
Shortages of penicillin make treatment for this preventable disease harder. “Clearly, stock outs are a problem. It means there is limited access to treatment and that is why rheumatic heart disease still persists,” explains Bongani Mayosi, president of the Pan-African Society of Cardiology (PASCAR).
A worldwide shortage
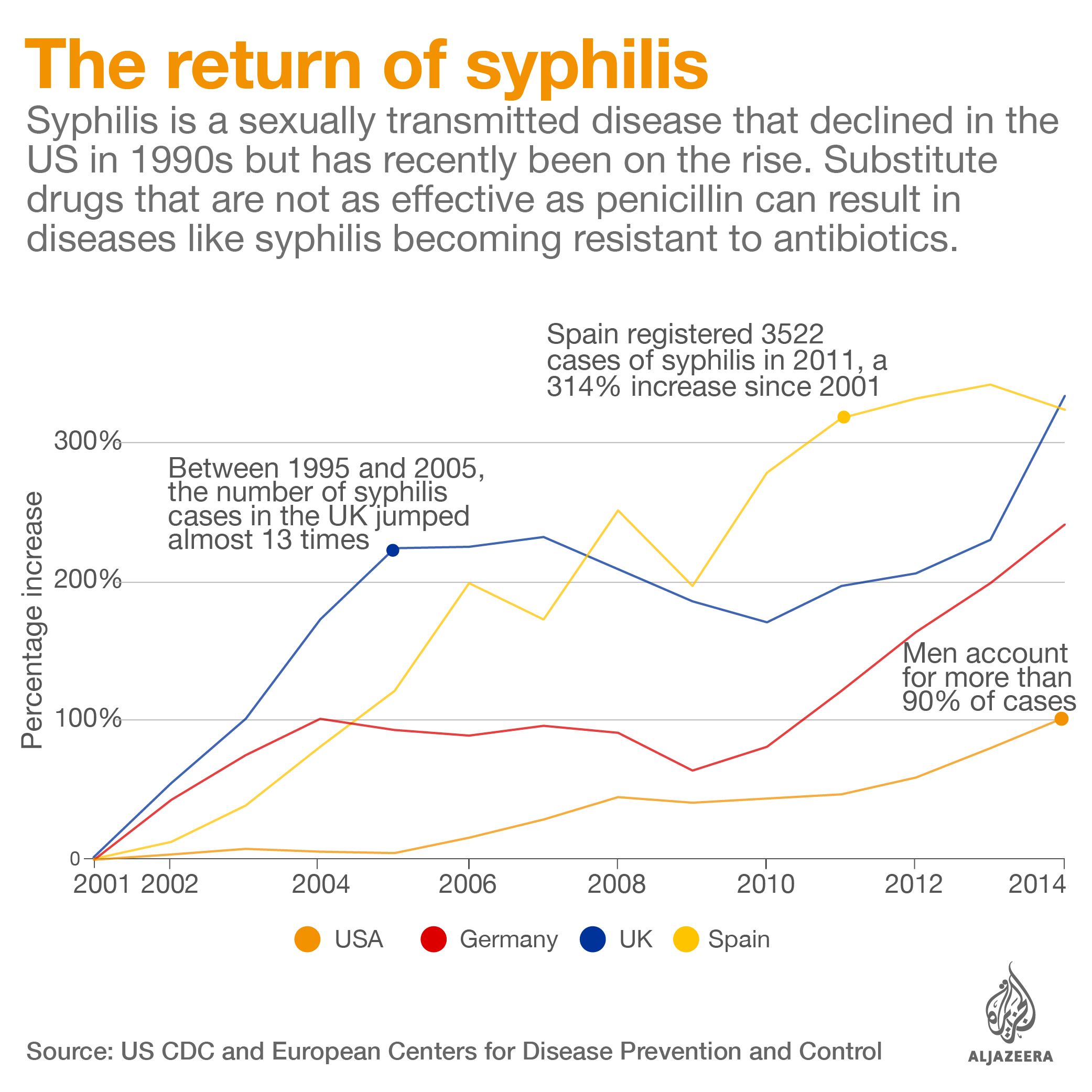
South Africa isn’t alone in its struggle. At least 18 countries, including the US, Canada, Portugal, France and Brazil, have faced shortages of benzathine penicillin G over the past three years, according to the World Health Organization.
In the US, a year-long shortage of benzathine penicillin G has made treating syphilis – a disease that is growing in prevalence – more difficult.
In Brazil, a three-year shortage of benzathine penicillin G came amid an outbreak of syphilis, a disease linked to severe malformation in babies. The antibiotic is the only drug that can kill the syphilis bacteria in the fetus.
“The Ministry of Health worked hard to recommend substitute drugs during the shortage, but there is no scientific basis that some of these antibiotics recommended actually pass through the placenta wall,” explains Maria Luiza Bezerra Menezes, medical coordinator of hospital Cisam in Recife, the capital of the northeastern state of Pernambuco.
Substitute medicines can also be more expensive. While a shot of benzathine penicillin G costs between $2.30 to $ 3.20 (R$7 to R$10) in Brazil, a dose of the antibiotic ceftriaxone costs double.
When untreated, syphilis during pregnancy can leave newborns blind and deaf and cause serious bone malformations. It is also linked to a high rate of stillbirths and infant mortality.
“Babies born with syphilis demand days of care, generating huge expenses. And sometimes we don’t even have the right drug for treatment,” says Luciane Cerqueira, a doctor at Hospital Universitario Pedro Ernesto in Rio de Janeiro and author of a recent study on syphilis in pregnant women in the city.
In an attempt to tackle the shortage, Brazil last year imported an emergency stock of 2.7 million vials of benzathine penicillin G.
But, despite such initiatives, doctors still struggle to source the antibiotic.
The effect of this is seen in hospitals across Brazil. Debora SM, a 21-year-old resident of Recife, Brazil who asked that her full name not be disclosed, gave birth to a baby with neurosyphilis in mid-February. The young mother and her son could have been treated with one shot of penicillin, but despite making numerous visits to her local clinic, Debora had no antenatal screening tests during her pregnancy as there were no doctors in the facility to see her.
![Doctors collect cerebral fluids from the spine of Debora's baby, born with neurosyphilis, to assess his condition [Courtesy of Heudes Regis]](/wp-content/uploads/2017/05/e64581e9eedb4f25ba9326c8535dc3f6_18.jpeg)
Debora only found out that she and her baby had syphilis at the time of delivery. “I got sad because my son was not born healthy,” she says. Her baby was treated with penicillin for more than 10 days and has endured painful tests to assess the severity of his condition. “Every time I see the injection I cry,” she says.
Over the next 18 months, both Debora and her son will need to regularly visit the health facility for tests. Only then will doctors be able to assess whether the baby has been left with any malformation and if the syphilis bacterium is no longer present in his central nervous system and brain.
Why is there a shortage?
Just four companies produce the active ingredient for penicillin – a drug that changed modern medicine 76 years ago – and because the medicine offers little profit, those companies keep production levels low.
As penicillin has been used to treat diseases, such as syphilis and rheumatic heart disease, which disproportionately affect poorer countries, the extent of the demand for the drug also isn’t always clearly captured.
“There is a market failure in the penicillin sector: there is a demand, but it comes from the poor,” explains Ganesan Karthikeyan, a cardiologist at the All India Institute of Medical Sciences in New Delhi, India.
India has the highest number of deaths caused by rheumatic heart disease, with 111,000 fatal cases in 2015, according to WHO Global Health Estimates. But, despite the number of people affected, the supply of penicillin, the drug that can stop the disease, has been irregular in the country for the past 15 years.
![A private doctor in New Delhi, India, sees a young patient complaining of a sore throat [Courtesy of Anshul Gupta]](/wp-content/uploads/2017/05/dd068591993c4350826b323ed4a2e153_18.jpeg)
In the case of syphilis, 5.6 million people are infected around the world every year, but that “is a small market from a pharmaceutical point of view,” explains Andy Gray, a consultant pharmacist and senior lecturer in pharmacology at the University of KwaZulu-Natal, South Africa.
The WHO has estimated that a shot of penicillin could have saved more than 53,000 babies from 30 countries who died from syphilis acquired in the womb in 2012.
Another factor that contributes to shortages is the fragmentation of the production process. Most pharmaceutical companies source the raw materials and the core drug ingredient – called API – from other companies, then formulate the final medicine, package and sell it worldwide. So a disruption in one company in this chain can impact the global supply.

“If a manufacturer of the active pharmaceutical ingredient leaves the market, this affects the producers of the finished product, which may cause delays in production and affect countries sourcing the drug,” says Maggie Savage, from the New Market Opportunities Team at Clinton Health Access Initiative (CHAI), which reviewed the global availability of benzathine penicillin G last year.
Over the past decade, a few companies have left the market looking for more profitable products.
“There is no money in penicillin, so companies will not produce it,” says New Delhi doctor Amit Sengupta, global coordinator of People’s Health Movement, a global network of grassroots health activists, civil society organisations and academic institutions.
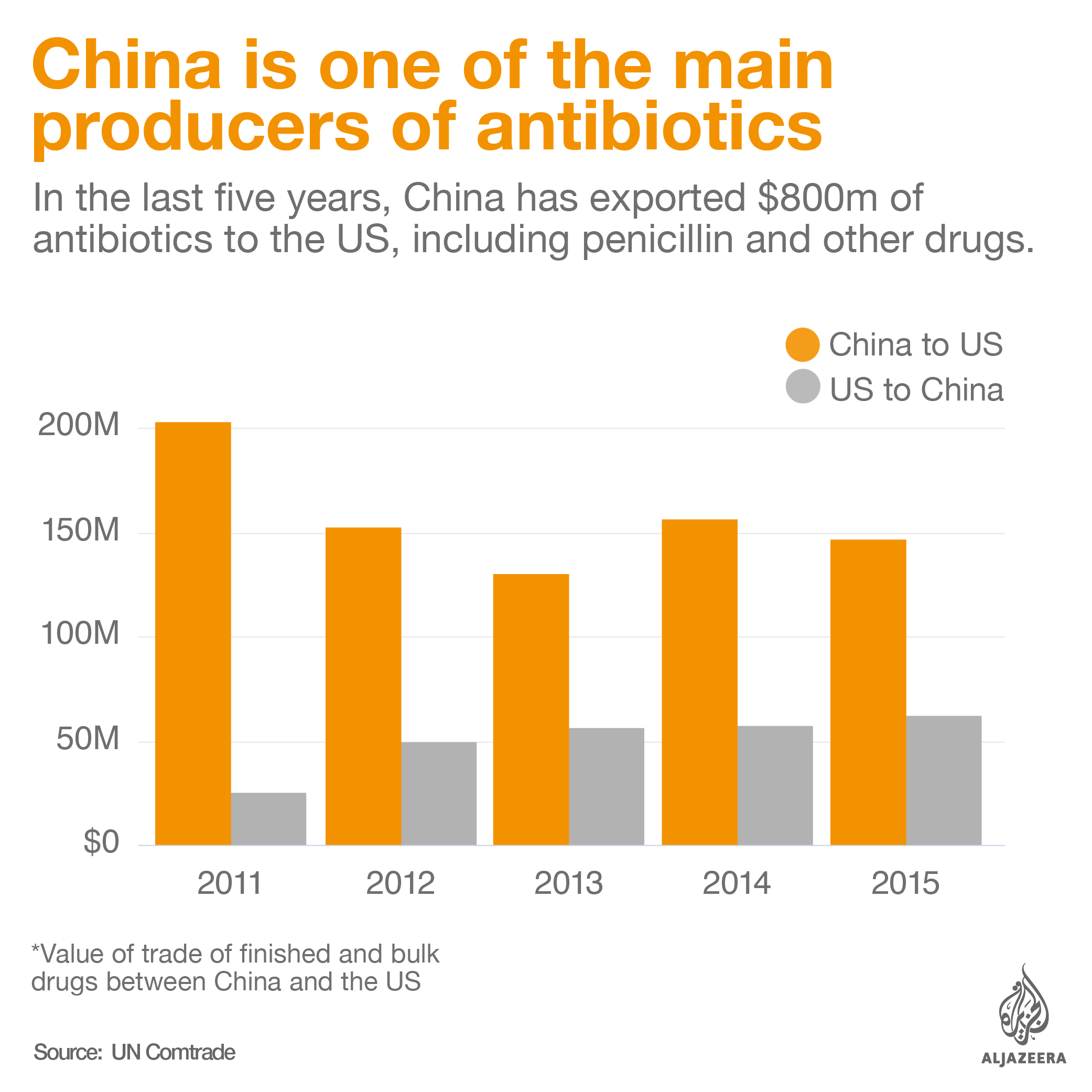
Turning to China
![A newborn infected with syphilis receives a shot of penicillin in a hospital in Rio de Janeiro, Brazil[Courtesy of Thiago Facina]](/wp-content/uploads/2017/05/ccb0e2fa7f3a4abf829bbc4018d1656e_6.jpeg)
Countries hard hit by the shortages have turned to Chinese companies they had previously avoided buying medicines from as a way of ensuring access to this essential medicine.
Last July, Brazil exempted a Chinese manufacturer, North China Pharmaceutical Group Semisyntech Co. Ltd, from a key local drug registry, intended to guarantee that drugs sold in the country are safe and efficient.
The waiver benefited three Brazilian drugmakers, including Laboratorio Teuto, a local partner of Pfizer, allowing them to import the unregistered active pharmaceutical ingredient (API) from the Chinese manufacturer.
The exemption was granted seven months after North China Pharmaceutical Group Semisyntech Co. Ltd’s requests to sell benzathine penicillin G in Brazil were denied by the country’s drug agency on the grounds of non-compliance with good manufacturing standards.
Prior to that, “critical deficiencies” were found in the Chinese plant by European authorities.
During a visit by France’s drug agency in November 2014, inspectors found falsification of documents, lack of data integrity in the quality control laboratory and risk of contamination in the medicines assembled in the plant, which is located in the city of Shijiazhuang, Hebei province. The French authority recommended that the company to be prohibited from supplying penicillin to EU members.
![A nurse holds a vial of benzathine penicillin G formulated in Brazil by Laboratório Teuto/Pfizer [Courtesy of Thiago Facina]](/wp-content/uploads/2017/05/819ba4ebcdce4ddaa1e90f031a635899_18.jpeg)
Following the inspection, the manufacturer also lost various certificates of suitability issued to drugs that meet high quality standards. Hong Kong, Ethiopia and Liberia recalled vials of penicillin made with ingredients from the plant and distributed by French drugmaker Laboratoires Panpharma, which sells antibiotics to more than 80 countries.
In March, after another inspection by the EU, North China Pharmaceutical Group Semisyntech Co. Ltd received a certificate of good manufacturing practices – but it was valid only for substances for veterinary use.
In a statement, Brazil’s Ministry of Health said it has been encouraging local companies to manufacture penicillin. Neither Brazil’s Ministry of Health nor the country’s drug agency, Anvisa, replied to questions about the safety of the unregistered ingredients. North China Pharmaceutical Group Semisyntech Co. Ltd has not responded to requests for a comment.
“Penicillin production is difficult,” says consultant pharmacist Gray. “This is a product with no alternatives and you rely on a few huge global suppliers.”
Why aren’t more manufacturers producing penicillin?
Three of the four companies that still produce the active pharmaceutical ingredient for benzathine penicillin G are located in China. These are North China Pharmaceutical Group Semisyntech Co. Ltd, CSPC Pharmaceuticals Group Ltd. and Jiangxi Dongfeng Pharmaceutical Co.. The fourth company is Austria-based Sandoz GmbH.
![A vial of benzathine penicillin G produced by Pfizer Limited in the medicine cabinet of a doctor in New Delhi. Almost all the penicillin in the country comes from China [Courtesy of Anshul Gupta]](/wp-content/uploads/2017/05/af798645f2ba4d51b1f2dbf3181aafe2_18.jpeg)
These companies produce only 20 percent of what they could because benzathine penicillin G is “off patent, offers little profit and because demand data is extremely limited,” according to the WHO. The medicine’s cheap selling price also makes manufacturers disinclined to enter the market.
With so few producers, drugmakers say they do not have many options when it comes to sourcing the drug’s ingredients.
Two years ago, while Portugal was struggling with a shortage of benzathine penicillin G, Portuguese drugmaker Laboratorios Atral SA turned to China after its former European supplier changed the presentation of the active substance – which Atral says made it incompatible with its formulation process.
Atral says none of the companies they evaluated in China had the full set of documents demanded by the the European Union, such as a certificate of suitability or the Active Substance Master File (ASMF), where a producer details its manufacturing process.
But, with no other options, the European drugmaker assisted the manufacturer in bringing together a package of information in line with EU legislation. Atral says it has audited the Chinese manufacturer, whose name it hasn’t disclosed, to ensure their standards.
“It is not easy. There are a few producers and, of those that are in the market, one is banned and the others don’t have complete documentation,” says Eduardo Oliveira, regulatory affairs director of Atral.
The company’s penicillin brand Lentocilli S is sold to at least five other countries.
South Africa last year also sourced more than 242,000 vials of unregistered benzathine penicillin G from North China Pharmaceutical Group Corp (NCPC), the parenting company of Semisyntech, through an emergency scheme.
Even India, a leading pharmaceutical producer, outsources almost all production of penicillin G to China, according to the Indian Drug Manufacturers’ Association (IDMA). “India became unviable as China’s price is too low to compete with,” says Ashok Kumar Madan, executive director of IDMA.
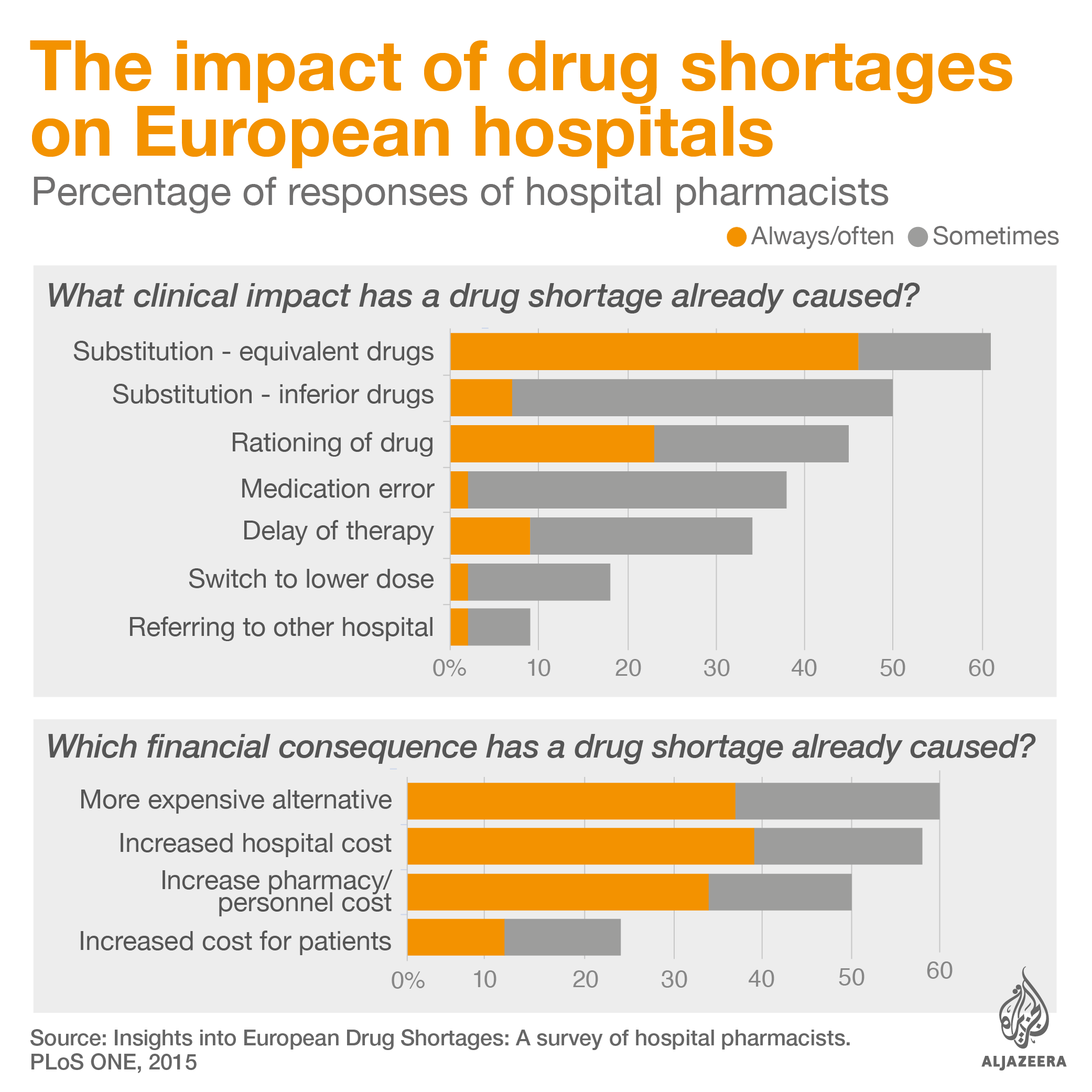
Global reliance on a few manufacturers can compromise stability of supply as manufacturing delays or failure in one of these sites can affect several countries and millions of patients at once. Shortages mean sick people are sometimes treated with less efficient and more expensive drugs. According to a 2015 survey with European hospital pharmacists, half said patients were given inferior drugs during shortages. More than a third said stock outs led to medication errors.
And stock outs are not rare. “Hospital pharmacists in Europe will usually face difficulties in sourcing a medicine that is not immediately available in their country,” notes Steve Glass, chief commercial officer of Clinigen for North America and Europe, a company that supplies hospitals with medicines.
The regulators’ dilemma
Such a small number of manufacturers also creates dilemmas for regulators, who have limited powers to restrict supply from some manufacturers, even when critical deficiencies have been found on their sites, fearing shortages of life-saving medicines.
According to the European Medicines Agency (EMA), in the past regulators have had to leave poor-quality drugs on the market to prevent stock outs of essential medicines. “Very difficult risk-benefit judgements had to be made between poor quality processes or product, or no product at all,” said the agency in a 2012 report.
The agency added that on occasion, “the ability of regulators to take action against a manufacturing site was restricted”, even when companies didn’t comply with quality standards, in order to avoid shortages.
Problems with manufacturing quality have intensified over recent decades as pharmaceutical companies increasingly source drug ingredients from external manufacturers, sometimes located far from where they operate. Experts warn that this practice can increase the risk of bad quality medicines and that a compliant supply chain is essential.
“Compliance with existing product safety regulations is clearly key to ensuring that pharmaceuticals are effective and do not harm patients’ health,” says Natasha Hurley, campaign manager from Changing Markets Foundation, which has investigated the supply chain of drug companies over the last years.
Lack of transparency is another issue. Companies rarely disclose who makes the drugs they sell, claiming the information is a matter of confidentiality. But poor visibility of the companies who actually produce drugs can make quality control more difficult.
In the case of penicillin, where almost all global production is outsourced to third-party producers, the issue is even more urgent. “The market itself is very fragmented. It has been a challenge to know who is really making the drug,” says Rosemary Wyber, deputy director of RhEACH, a group that coordinates global actions to tackle rheumatic heart disease, who coordinated a report on the availability of benzathine penicillin G last year.
“The more we learn from the clinical side and the more we try understanding the manufacturing side, we realise this is not a transparent market at all.”
Are penicillin shortages contributing to antibiotic resistance?
![A pregnant woman goes through a syphilis test in a health clinic in Rio de Janeiro, Brazil, hit by an outbreak of the disease and by a shortage of penicillin [Courtesy of Thiago Facina]](/wp-content/uploads/2017/05/ce32c3e74d12473ca1857f550a1a0ab1_18.jpeg)
Doctors are becoming increasingly concerned about antibiotic resistance fuelled by the use of substitute drugs that are not as effective as penicillin.
Penicillin was once considered a “miracle drug”, but global shortages are pushing doctors towards substitute medicines such as azithromycin – a drug increasingly found inefficient against certain strains of the bacterium known as Treponema pallidum, which causes syphilis.
Genetic mutations that make the syphilis bacteria resistant to a family of antibiotics called macrolides, which includes drugs erythromycin and azithromycin, have been documented across the world over recent decades.
The extensive use of these drugs is one of the reasons why resistant syphilis has emerged, according to researchers from the University of Zurich, authors of a paper about resistant strains of the disease. Penicillin is the first-line therapy against syphilis, but macrolides are used when penicillin is not available or in cases of penicillin allergy.
While antibiotic resistance is a natural process, shortages of first-line medicines can increase the risk.
“If penicillin is not available, clinicians are forced to use second-line drugs, such as macrolides,” says Lola Stamm, a microbiologist at University of North Carolina and author of a recent article on resistant syphilis bacteria.
“Azithromycin works for syphilis as long as the syphilis bacteria does not have a mutation that confers resistance to the drug. The problem now is that azithromycin-resistant Treponema pallidum has emerged and spread.”
While benzathine penicillin G is a narrow spectrum antibiotic that kills specific germs – such as the ones that cause syphilis – azithromycin is broad spectrum. This means that the drug targets multiple species of bacteria in the human body, including non-harmful ones, such as those that naturally live in the patient’s gut.
“This brings selection pressure among non-harmful bacteria and in turn these bugs develop various resistance mechanisms against broad spectrum antibiotics,” explains Vikas Manchanda, assistant professor at the Maulana Azad Medical College, in New Delhi, India.
“This normal flora later transfers resistance to harmful bacteria and other germs in the patient’s own body as well as in the environment where they are shed,” adds Manchanda.
Broad spectrum drugs can also destroy essential bacteria naturally present in the human body, leaving a patient vulnerable to harmful germs.
Shortages of benzathine penicillin G also push doctors into using antibiotics from the macrolide family to control group A strep, the germ related to rheumatic heart disease.
But over the past decade, researchers have identified macrolide-resistant group A strep in countries such as Argentina, France, Canada, the US and China – and the prevalence of resistant strains has been increasing.
Macrolides have been used since the 1950s as an alternative therapy against these bacteria. However, azithromycin’s effectiveness in controlling rheumatic heart disease and avoiding further heart damage caused by group A strep is unknown and there is now evidence that the drug fails against resistant strains.
Because of these, the medicine should be kept as a second-line option, but shortages of penicillin exacerbate its use.
“When injectable penicillin is not available, tablet antibiotics such as macrolides are used sometimes. For patients, this implies that tablets are equivalent to regular antibiotic injections. Unfortunately this is not the case – tablet antibiotics are less effective and may have unintended effects on antimicrobial resistance,” says Wyber of RhEACH.
“Macrolides in particular are concerning because they can drive resistance in group A strep themselves as well as other organisms like pneumococcus,” she adds.
Resistant bugs are a major public health threat worldwide. A recent report commissioned by the UK government estimated that 700,000 lives are lost annually to drug resistance.
The report warns that, if not addressed now, resistant organisms will kill as many as 10 million people a year by 2050, with a loss of up to $100 trillion to the global economy. This scenario has prompted the World Health Organization to name antibiotic resistance as as one of the most important public health threats of the 21st century.
No money in the market
The evolution of bacteria to adapt to the drugs that are meant to kill them was documented soon after the introduction of the first antibiotics. For example, penicillin-resistant strains of Staphylococcus aureus, linked to life-threatening diseases such as pneumonia and meningitis, began to rise as penicillin became widely available after World War II.
More recently, resistance has become a growing problem as the discoveries of new antibiotics have slowed down, while shortages of old and useful drugs are on the rise, limiting the options available for treatment.
According to the Centers for Disease Control and Prevention (CDC), only nine new antibiotics were developed and approved in the US between 2005 and 2014 – a third of the number of drugs approved in the 1980s.
Fewer antibiotics are being developed because these drugs now offer lower margins than other medicines, such as those targeting rare and chronic diseases. A 2011 study from the London School of Economics estimated that the cost of developing a new drug ranges from $802 million to $1.7 billion. But while the net present value – the relationship between the projected costs and revenues of a product – for injectable antibiotics stood at $100 million, the forecast for drugs to treat musculoskeletal disorders, such as arthritis, was over $1 billion.
Last year, of the 2,240 new drugs being developed only eight percent were from the anti-infectives family, which includes antibiotics, according to a report by QuintilesIMS Institute.
On top of fewer antibiotics being made, old drugs are increasingly under shortage, because they are cheap and offer little profit. However, ensuring access to these drugs is crucial for tackling the spread of resistance, argue experts.
“We need new antibiotics, but we also need to preserve the existing ones because they can save us right now,” says Céline Pulcini, who has coordinated a study on shortages of old antibiotics and resistance in 39 countries across North America and Europe.
The researcher and her team found out that several old medicines useful against resistant germs were not available in most of the countries surveyed in 2015, mainly for economic reasons. Benzathine penicillin G, which costs as much as $2 a vial, was unavailable in 20 of the nations surveyed.
“When you don’t have penicillin to treat syphilis you use ceftriaxone or macrolides, which are second-line drugs, and then the problem of resistance arises. That is not good for the patient in terms of efficacy and it is not good for the world because this selects more resistance,” says Pulcini.
Pulcini argues that the development of new drugs should be pursued together with ensuring that old medicines are available for patients. “There is a very strong focus on new drugs but almost nothing much is said about existing ones. And that is basically because there is no much money in this market. The price is so low that nobody is interested.”
“It must be a top priority for governments to solve this,” she adds. “We need new business models and incentives to have old antibiotics in the market, otherwise we will just lose these drugs.”
This project has been funded by the European Journalism Centre (EJC) via its Innovation Development Reporting Grant Programme.