US regulators approve 2nd COVID booster for those aged 50 and up
US has seen a small uptick in new COVID-19 cases during the past week, raising concern that infections could surge again.
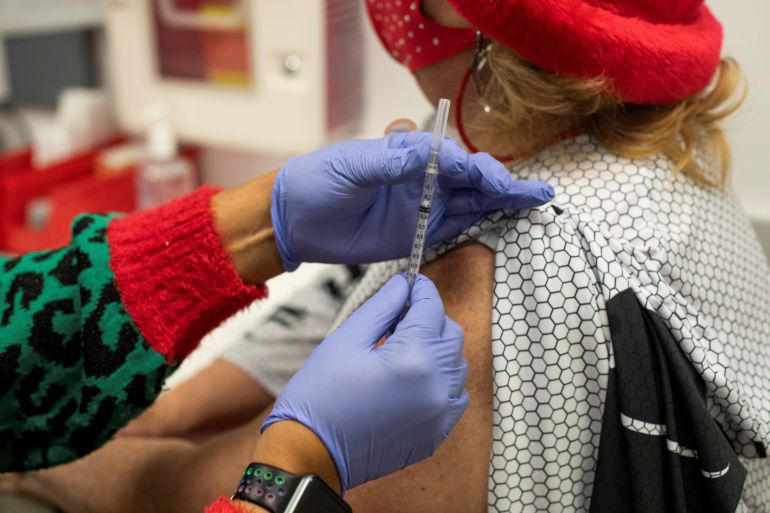
Regulators in the United States have authorised another COVID-19 booster shot for people aged 50 and older, amid concerns that coronavirus infections may begin rising again due to waning immunity to Omicron variants.
The US Food and Drug Administration’s (FDA) decision on Tuesday allows a fourth dose of the Pfizer or Moderna vaccines to be administered at least four months after the previous dose.
Keep reading
list of 3 itemsWhat we know so far about the new Omicron subvariant
Is the Omicron subvariant BA.2 more transmissable?
“Based on an analysis of emerging data, a second booster dose of either the Pfizer-BioNTech or Moderna COVID-19 vaccine could help increase protection levels for these higher-risk individuals,” top FDA official Dr Peter Marks said in a statement.
The US Centers for Disease Control and Prevention (CDC) later updated its recommendation in line with the FDA, saying the additional boosters were safe and would allow people to “increase their protection further”.
COVID-19 cases in the US have dropped sharply since a record surge in January, but have seen a small uptick during the past week, according to CDC data.

Until now, the FDA had cleared fourth doses only for people 12 and older who have severely weakened immune systems. The agency said this especially fragile group also can get an additional booster, a fifth shot.
Two vaccine doses and a booster still provide strong protection against severe disease and death, CDC data show.
The CDC also said on Tuesday that in line with new data, “adults who received a primary vaccine and booster dose of Johnson & Johnson’s Janssen COVID-19 vaccine at least 4 months ago may now receive a second booster dose using an mRNA COVID-19 vaccine”.
Tuesday’s announcements come at a time of great uncertainty in the pandemic, as COVID-19 cases in the US have dropped to low levels after the winter surge but as a sub-variant of Omicron known as BA.2 is spreading.
Meanwhile, vaccination rates have stalled. About two-thirds of Americans are fully vaccinated, but half of those eligible for a first booster have not gotten one.
Scientists and officials have debated for months if and when an additional booster shot would be needed as they parsed through data on how long protection from the vaccines and boosters would last.
“It’s not clear that now is the right time for people to get a fourth dose,” said Dr William Moss, executive director of the International Vaccine Access Center at the Johns Hopkins Bloomberg School of Public Health.

If there is a surge in cases in late fall or early winter, as is typical for respiratory viruses and influenza, an additional boost may then be needed, Moss said. The body’s neutralising antibodies spurred on by a fourth booster given now may decline in just a few months, he added.
It is also unclear whether young, healthy people will need a fourth shot. The study of Israeli healthcare workers cited by the FDA suggested that the fourth dose added little additional protection in the age group.
Officials in US President Joe Biden‘s administration have said that the government currently has enough vaccine doses to meet the demand for another round of booster shots for older Americans, even as funding for the US pandemic response has all but run out.
They said that unless Congress approves more spending, the government likely will not be able to pay for future inoculations, if they are needed, particularly if the vaccines need to be redesigned to target new variants.