US grants full safety approval to Pfizer COVID-19 vaccine
FDA decision paves the way for wider distribution of jabs and vaccine’s legal mandates in the US.

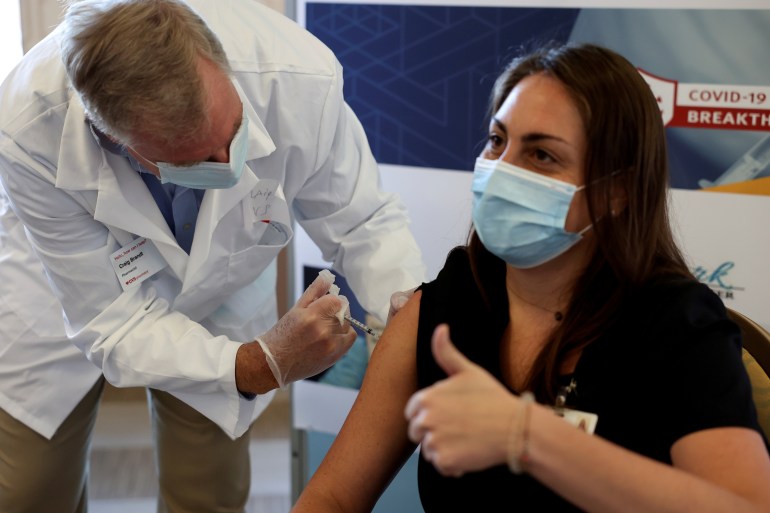
The United States Food and Drug Administration (FDA) has given full safety approval to Pfizer and BioNTech’s COVID-19 vaccine in a landmark decision that will allow leading US employers and universities to mandate jabs.
The FDA’s decision brings about the first US government licence of a vaccine against the coronavirus that causes COVID-19 which has killed more than 628,500 people in the US since the pandemic began, according to data from Johns Hopkins University.
Keep reading
list of 4 itemsBarbie debuts doll in likeness of UK COVID-19 vaccine developer
US to make COVID-19 vaccines mandatory for all US troops
US to send more COVID-19 vaccines to Mexico amid Delta surge
“With today’s FDA full approvals there’s another good reason to get vaccinated, so please get vaccinated,” said President Joe Biden, urging Americans who have not yet gotten vaccinated to get the jab.
The Pfizer vaccine, previously approved on an emergency basis, has been widely distributed inside the US and is being shipped by the US government to other nations.
“While millions of people have already safely received COVID-19 vaccines, we recognize that for some, the FDA approval of a vaccine may now instill additional confidence to get vaccinated,” said Janet Woodcock, the FDA’s acting commissioner.
The decision is for vaccines for people aged 16 and above. The FDA is still reviewing data for those aged between 12 and 15 and has not yet allowed the vaccine for younger children.
“Today’s milestone puts us one step closer to altering the course of this pandemic in the US,” Woodcock said in a statement.
The FDA’s approval is based on updated data from the company’s clinical trial.
“Based on the longer-term follow-up data that we submitted, today’s approval for those aged 16 and over affirms the efficacy and safety profile of our vaccine at a time when it is urgently needed,” Albert Bourla, CEO of Pfizer, said in a statement.
The Pfizer-BioNTech vaccine will now be marketed under the name Comirnaty, the FDA said.
The vaccine contains genetic material, messenger RNA, that allows the human body to generate an immune response by mimicking the virus, previously unseen in humans.
Some US cities, including New York and San Francisco, have already imposed rules requiring vaccination to dine at restaurants or work in government offices, among other activities.
The FDA decision provides the legal underpinning for the US military’s mandate requiring all service personnel to get the vaccine that will serve as a model for other states and large public organisations.
Even as the first round of vaccines are still being distributed, US health officials are now recommending that all Americans get booster jabs to shore up their protection amid the surging Delta variant and evidence that the vaccines’ effectiveness is falling.
“Recent data makes it clear that protection against mild and moderate disease has decreased over time,” Dr Vivek Murthy, the US surgeon general, said on Wednesday.
Some Americans have been resisting the vaccines, leading to large outbreaks of the Delta variant among the unvaccinated in some states, like Texas and Florida.