Infographic: Vaccinating children against COVID
More than 100 countries around the world are vaccinating children against COVID-19.

More than 100 countries around the world have extended their COVID-19 vaccination drives to children.
According to a report from UNICEF, which analysed 115 million confirmed COVID-19 cases from 105 countries, people under 20 account for 16 percent of reported cases.
Keep reading
list of 4 items‘Dishonest competition’: Putin lashes out at G20 over vaccines
North American leaders to curb methane, boost vaccines: Officials
US prepares to roll out COVID vaccines for children aged 5 to 11
While many children who do catch COVID have few to no symptoms, those with underlying health conditions may be at increased risk of severe illness.
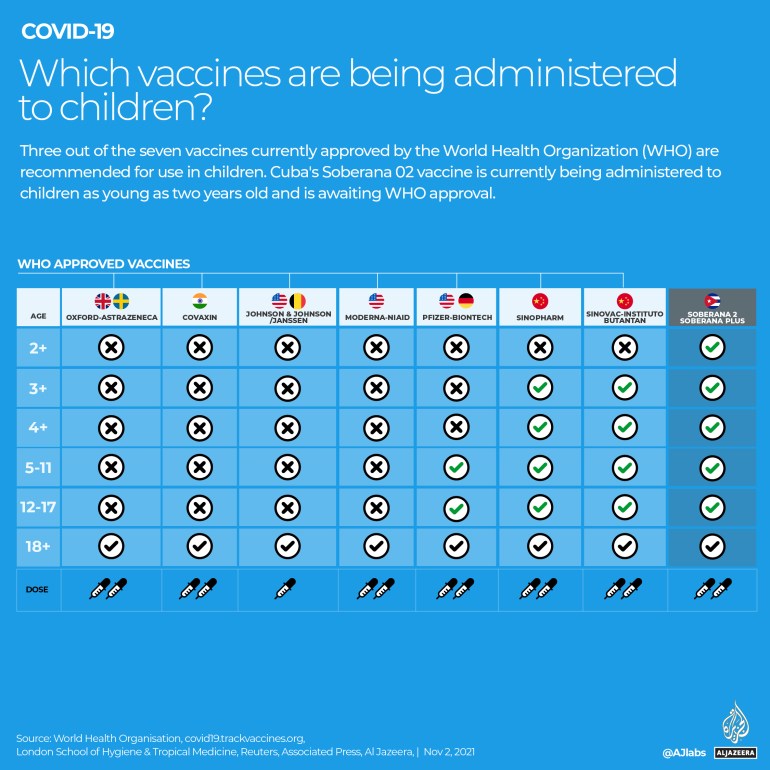
Three vaccines that have been approved by the World Health Organization (WHO) are being administered to children in various countries: the Pfizer-BioNTech vaccine, the Sinopharm shot and the Sinovac vaccine.
While some countries are giving children and adolescents the full two-dose course of the Pfizer-BioNTech vaccine, others are giving a single dose.
According to the London School of Hygiene & Tropical Medicine, there are 20 vaccine candidates in clinical testing for those under 18.
Asia
China has begun inoculating children aged three and above with its own CoronaVac vaccine, produced by Sinovac Biotech.
Taiwan has temporarily suspended giving second doses of the Pfizer-BioNTech vaccine to children and adolescents between 12 and 17 years over concerns from the risk of myocarditis (inflammation of the heart muscle) and pericarditis (inflammation of the outer lining of the heart), according to media reports.
In August, India’s drug regulator approved Zydus Cadila’s needle-free ZyCoV-D vaccine for those aged 12 and above. It is the world’s first DNA-platform vaccine for COVID-19, but its rollout has not begun. Bharat Biotech, the makers of the domestically manufactured Covaxin vaccine, sought permission for emergency use in children aged 12 and over, but regulators have yet to announce a decision. Vaccination for some groups of children could begin in January, news reports say.
Pakistan is currently administering the Pfizer-BioNTech vaccine to children over age 15, and the Chinese Sinovac and Sinopharm vaccines to children over 12.
Africa
South Africa began the immunisation of children aged 12 to 17 in October. The government is aiming to inoculate about six million adolescents in this age group with a single dose of the Pfizer-BioNTech vaccine.
Morocco, one of the first African countries to offer shots to children and adolescents, began vaccinating those between ages 12 and 17 in September. It is using the Pfizer-BioNTech and Sinopharm vaccines with the aim of immunising at least three million children.
Europe
The European Union’s health regulator, the European Medicines Agency (EMA), in May approved the use of the Pfizer-BioNTech vaccine for use in children over the age of 12.
In Germany, as of November 23, some 45 percent of the 12 to 17 age group had been fully vaccinated with the Pfizer-BioNTech vaccine.
In Norway, which is not part of the EU, the Pfizer-BioNTech vaccine has been approved for those aged 12 to 15. But health authorities have paused the rollout of second doses, partly due to a rare side effect linked to heart inflammation.
In the United Kingdom, which is not part of the EU, 12 to 15 year olds are being offered a single Pfizer-BioNTech jab.
The EU regulator is evaluating an application to extend the use of the vaccine to children aged five to 11. It is expected to make an announcement next month.
Latin America
In September, Cuba began rolling out vaccines for children as young as two years old, with the domestically produced Soberana-02 and Soberana Plus vaccines.
In Venezuela, authorities announced in early November that vaccinations were under way for children as young as two years old, using the Cuban Soberana-02 shot.
Brazil’s health regulator Anvisa has approved the use of the Pfizer-BioNTech vaccine in children from age 12 to 17 and the government has encouraged local authorities to prioritise those children and adolescents with comorbidities.
Middle East
The United Arab Emirates began its vaccination drive in August in children aged three to 11 with the Sinopharm vaccine. In November, Emirati authorities approved the Pfizer-BioNTech vaccine for children between five and 11.
In October, Bahrain authorised the use of the Sinopharm shot for children ages three to 11. This month, it approved the use of the Pfizer-BioNTech for children aged five to 11.
United States
In May, US health authorities approved the Pfizer-BioNTech vaccine for use in children from the ages of five to 17.
Since the beginning of the pandemic, children represented 16.9 percent of all confirmed cases, according to the American Academy of Pediatrics. In the week ending November 18, children made up 25.1 percent of reported cases. Children make up 22.2 percent of the US population.