Coronavirus vaccine race: US ‘Operation Warp Speed’ explained
The federal initiative aims to fast-track development of drugs to combat COVID-19 in the United States.

As nations around the world race to develop a vaccine to fend off COVID-19, the United States is rushing to be at the forefront of the battle.
In early April 2020, the administration of President Donald Trump announced Operation Warp Speed, a flagship federal initiative to fast-track the development of drugs to combat the novel coronavirus. The US government has so far invested more than $5bn in more some 30 research projects for the development of vaccines, diagnostics and treatments.
Keep reading
list of 4 itemsWill the US unemployment rate continue at historic lows?
Mexico’s teachers seek relief from pandemic-era spike in school robberies
‘A bad chapter’: Tracing the origins of Ecuador’s rise in gang violence
At least 4.2 million Americans have been infected by the new coronavirus, and more than 146,000 have died from the disease, according to Johns Hopkins University.
What is Operation Warp Speed?
According to the US Health and Human Services, Operation Warp Speed aims to deliver 300 million doses of a safe and effective vaccine by January 2021.
Under the initiative, the government will speed development and buy vaccines – before they are deemed safe and effective – so that the medication can be in hand and quickly distributed once the FDA approves or authorises its emergency use after clinical trials.
Americans will receive the vaccine for free, the companies said.
Which companies have been funded so far to develop vaccines?
1. Moderna
Moderna announced on July 27 that it had started a late-stage trial to test the effectiveness of its vaccine candidate – the first such trial under Operation Warp Speed and that last hurdle before regulatory approval.
Moderna’s study will test the response to the vaccine in 30,000 adults who do not have the respiratory illness. The company, which has never brought a vaccine to market, has received nearly $1bn from the US government, which has chosen it as one of the first to enter large-scale human trials.
The late-stage trial is designed to evaluate the safety of Moderna’s mRNA-1273 and to determine if the vaccine can prevent symptomatic COVID-19 after two doses. The study also seeks to answer if the vaccine can prevent death caused by COVID-19, and if just one dose can prevent symptomatic COVID-19.
Moderna said it is on track to deliver about 500 million doses a year, and possibly up to one billion doses a year, beginning 2021.

2. Pfizer/BioNTech SE
Pfizer Inc and German biotech BioNTech SE, have been awarded $1.95bn in US government funding to develop a vaccine that utilises the chemical messenger RNA to instruct cells to make proteins that mimic the surface of the coronavirus, which the immune system sees as a foreign invader and mounts an attack. Although the technology has been around for years, there has never been an approved messenger RNA (mRNA) vaccine.
The company aims to make up to 100 million doses by the end of 2020 and potentially more than 1.2 billion doses by the end of 2021. The vaccine has already shown promise in early-stage small studies in humans, producing the type of neutralising antibodies needed to fight the virus. In those trials, subjects received two doses of the vaccine.
3. Novavax
On July 7, the US government awarded Novavax $1.6bn for its potential vaccine. The company aims to start mid-stage trials in August or September, with Phase III testing starting in October, with the aim of delivering 100 million doses by January. The Novavax vaccine works in conjunction with an adjuvant – a substance that boosts the immune response to help the body build a robust defence against the virus.
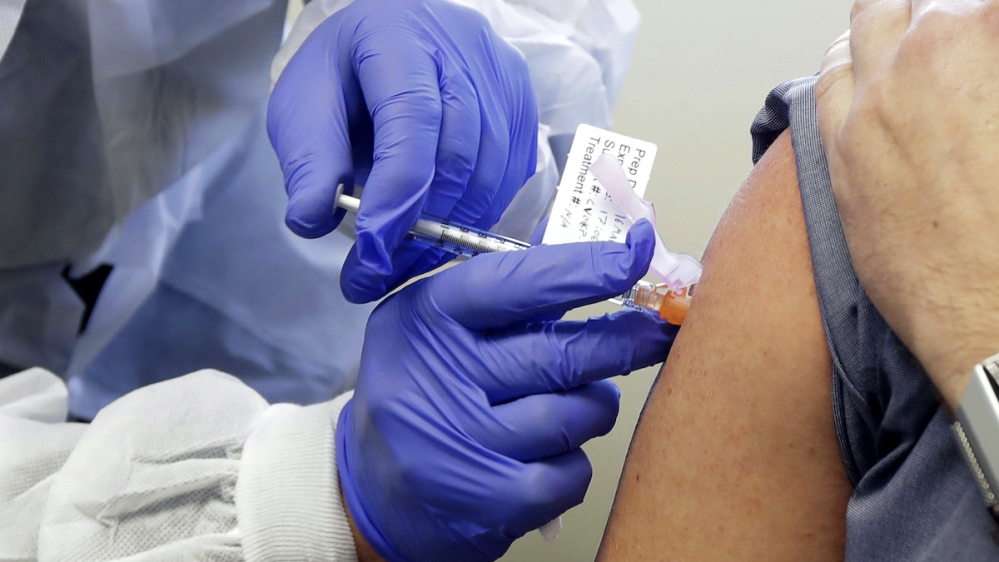
4. AstraZeneca
Developed by the University of Oxford, AstraZeneca has received $1.2bn in US federal funding. The experimental vaccine is composed of a recombinant viral vector vaccine that uses a weakened version of a common-cold virus that encodes instructions for making proteins from the novel coronavirus to build immunity.
5. Johnson & Johnson
With a $456m investment from the US government, Johnson & Johnson (J&J) is aiming to begin in September a late-stage study of its experimental vaccine, lagging other companies on this list.
J&J’s candidate is developing what is known as a viral vector vaccine, which relies on a non-replicating virus to shuttle genetic instructions into the body’s cells. Those instructions tell the cell to make viral proteins that would train the immune system to fend off real infections in the future.
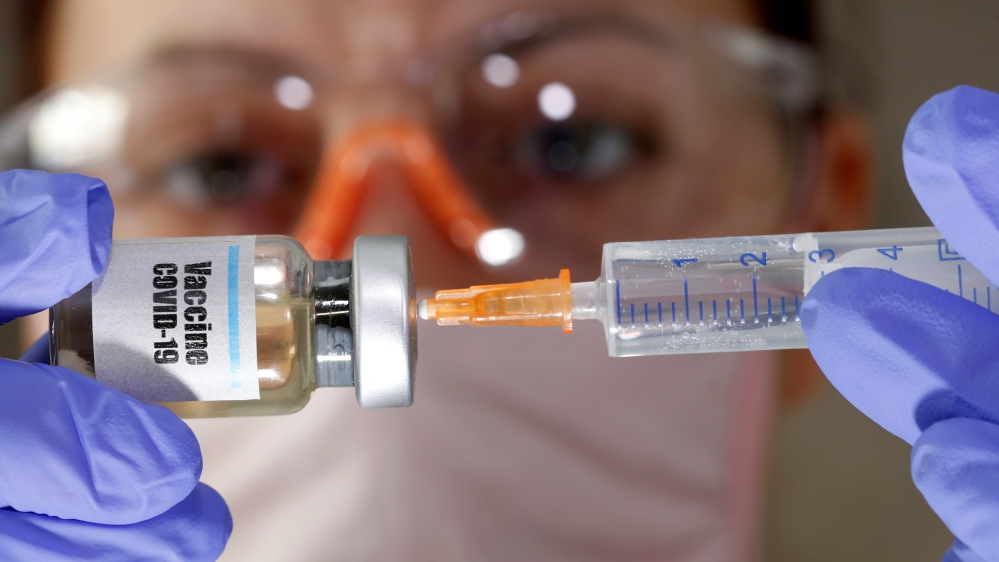
6. Merck & Co
Merck & Co received $38m in US federal funding to develop a recombinant COVID-19 vaccine that is based on the same technology used in the company’s Ebola vaccine. Human trials are expected sometime this year.
7. Sanofi SA
With some $30.7m in US government funding, Sanofi is developing a recombinant SARS-CoV-2 Protein Vaccine, based on FDA-licensed seasonal influenza vaccine. The company plans to enrol thousands of subjects globally for early-stage testing.