French breast-implant case may turn criminal
Government to open complaint of criminal fraud and deceit against Jean-Claude Mas and his company partners.
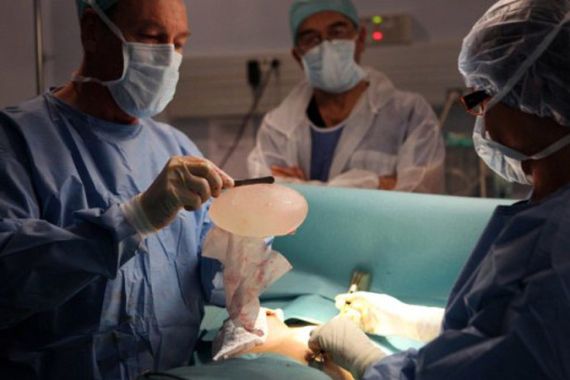
The French government will file a case alleging fraud and deceit against the makers of potentially risky breast implants, as calls of action against the company’s founder mounted.
The head of France’s state medical insurance, Frederic Van Roekeghem, told AFP Saturday his agency would be laying complaints within days alleging criminal fraud as well as seeking compensation.
Keep reading
list of 4 itemsDeadly Sahel heatwave caused by ‘human-induced’ climate change: Study
Woman, seeking loan, wheels corpse into Brazilian bank
UK set to ban tobacco sales for a ‘smoke-free’ generation. Will it work?
“We expect a case to be opened very quickly and the responsibilities of the company management clearly revealed,” he said.
Jean-Claude Mas, 72, the founder and CEO of French company Poly Implant Prothese (PIP) has not been seen or heard of in public since the scandal potentially affecting 300,000 women around the world broke.
His company is accused of using sub-standard industrial silicone in some of its implants, which were sold globally before being taken off the market in 2010.
“It’s obvious we have to find him (Mas) and those who had an interest in this company,” French Health Minister Xavier Bertrand said. “By not using the specified product, they sought to make money, and this is the worst thing, out of women’s health. They have to answer for their actions.”
But global police agency Interpol said that a “red notice” it issued seeking Mas’s arrest had nothing to do with his role at PIP.
France offered on Friday to pay for 30,000 women to have their PIP implants removed because of the risk that the products could rupture and leak industrial-grade silicone.
Francois Godineau, a top official in the French national health service, estimated it could deplete French government coffers by $78m.
Bertrand has recommended the unprecedented removal, saying it was a “preventive” measure and not urgent. French health officials said there was no link between the pre-filled silicone gel implants and nine cases of cancer among women implanted with them.
Some 30,000 women in France, and tens of thousands more in Britain, Italy, Spain, Portugal and other countries in Europe and South America have implants made by PIP. The implants in question were not sold in the US.
Drastic measure
Investigators say PIP, which pulled the implants from the market last year, used cheaper industrial silicone for the implants instead of medical silicone to save money.
One reason for the drastic measure is the uncertainty about the contents of the silicone gel used and the risks it poses to internal organs.
Also, standard mammograms and ultrasounds do not always indicate that an implant has ruptured, and many women may be walking around unknowingly with burst implants.
All breast implants are subject to rupture, especially as they get older, and patients are meant to be informed of the risks before implant surgeries.
But “these implants have a particular fragility” and appear to pose risks of rupture earlier in their life spans than other implants, Jean-Claude Ghislain of French health agency AFSSAPS, told a news conference on Friday.
Removal of the implants can require general anaesthesia and other risks associated with major surgery. The government recommendations say women who do not want to get them removed should be examined every six months.
Annie Mesnil, who had a PIP implant to replace a breast removed after cancer in 1999, said: “It’s not enough. They will pay for the removal of the implants, but they will not pay for the replacements.”
France’s state health care system normally pays for implants for medical reasons, but not for cosmetic implants. About 80 per cent of those with the PIP silicone implants have them for aesthetic reasons.