Doctor’s Note: Can dexamethasone treat COVID-19?
While a vaccine is being developed, researchers are trialling existing drugs that could help doctors treat coronavirus.
With the coronavirus pandemic tightening its grip on the world and economies grinding to a standstill, the race is on to find a vaccine. But while that could take up to 18 months, existing drugs are being fast-tracked to see if they can be effective in treating people with the disease and helping save lives.
With about 14 percent of COVID-19 patients needing to be hospitalised for respiratory problems, effective treatments are desperately needed.
Keep reading
list of 4 itemsLong COVID, rest, and helping our immune systems recover
Tumours disappear in all participants of small cancer drug trial
Can new Omicron subvariants evade vaccine immunity?
In March, the World Health Organization (WHO) announced a large global trial, called Solidarity, to find out whether any existing drugs can treat infections caused by COVID-19. Something on this scale has not been done before. The trial involves looking at scientific data from several countries, thousands of patients and hundreds of hospitals.
The idea is, instead of coming up with new drugs from scratch (which can take years), to see if drugs that are known to help with other conditions might also be effective in treating those with COVID-19.
Researchers are looking at certain drugs in particular, including lopinavir-ritonavir (also called Kaletra), used in the treatment of human immunodeficiency viruses (HIV). Other researchers have meanwhile trialled the steroid dexamethasone, which has yielded some promising results.
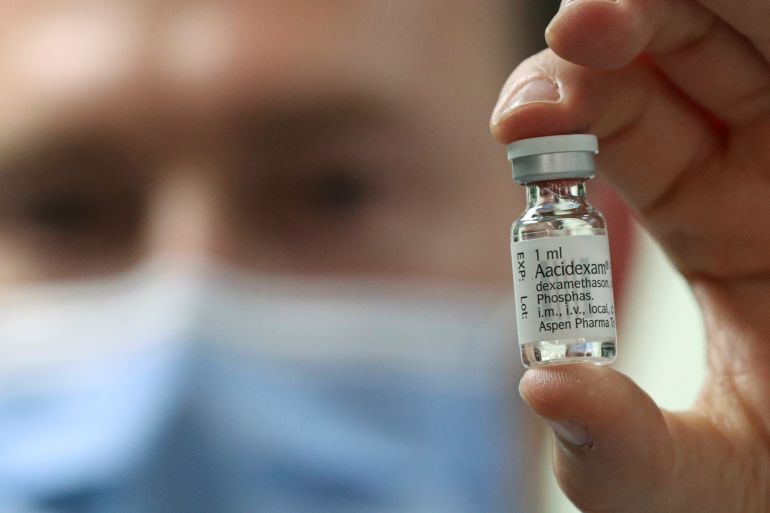
Dexamethasone
A recent announcement by a research team at Oxford University, England allowed for a glimmer of hope in what can only be described as a dark few months.
The drug dexamethasone had been trialled on around 2,000 patients who required hospital treatment as a result of COVID-19. The drug was shown to significantly reduce the number of deaths among those seriously ill with the coronavirus, so much so that the results have been fast-tracked and shared with the wider medical profession so other countries can benefit from these positive findings.
Dexamethasone is a steroid-based medication. It has long been used in the treatment of common conditions such as croup in children, asthma exacerbations, rheumatoid arthritis and for some conditions that cause brain swelling.
Part of the way it works is by suppressing the immune system. What we do know about patients that suffer the worst complications of COVID-19, such as breathing difficulties, is that their own immune system over- responds to the virus, so as well as attacking infected cells it also attacks healthy cells such as those found in the lungs.
Dexamethasone helps prevent this over-reaction so that only infected cells are targeted by the immune system.
The study showed that the drug reduced the number of deaths in coronavirus patients requiring a ventilator by one-third, and for those requiring oxygen, the number of deaths was reduced by one-fifth.
As the drug has been used for so long to treat other conditions, it is no longer on patent, which means no single pharmaceutical company has the right to it. As a result, it is relatively cheap to use and poorer countries, where the virus is having devastating effects, can also access it.
This is good news, but it is important to say that this does not prevent people from getting the virus and nobody should be buying or taking dexamethasone without medical supervision.
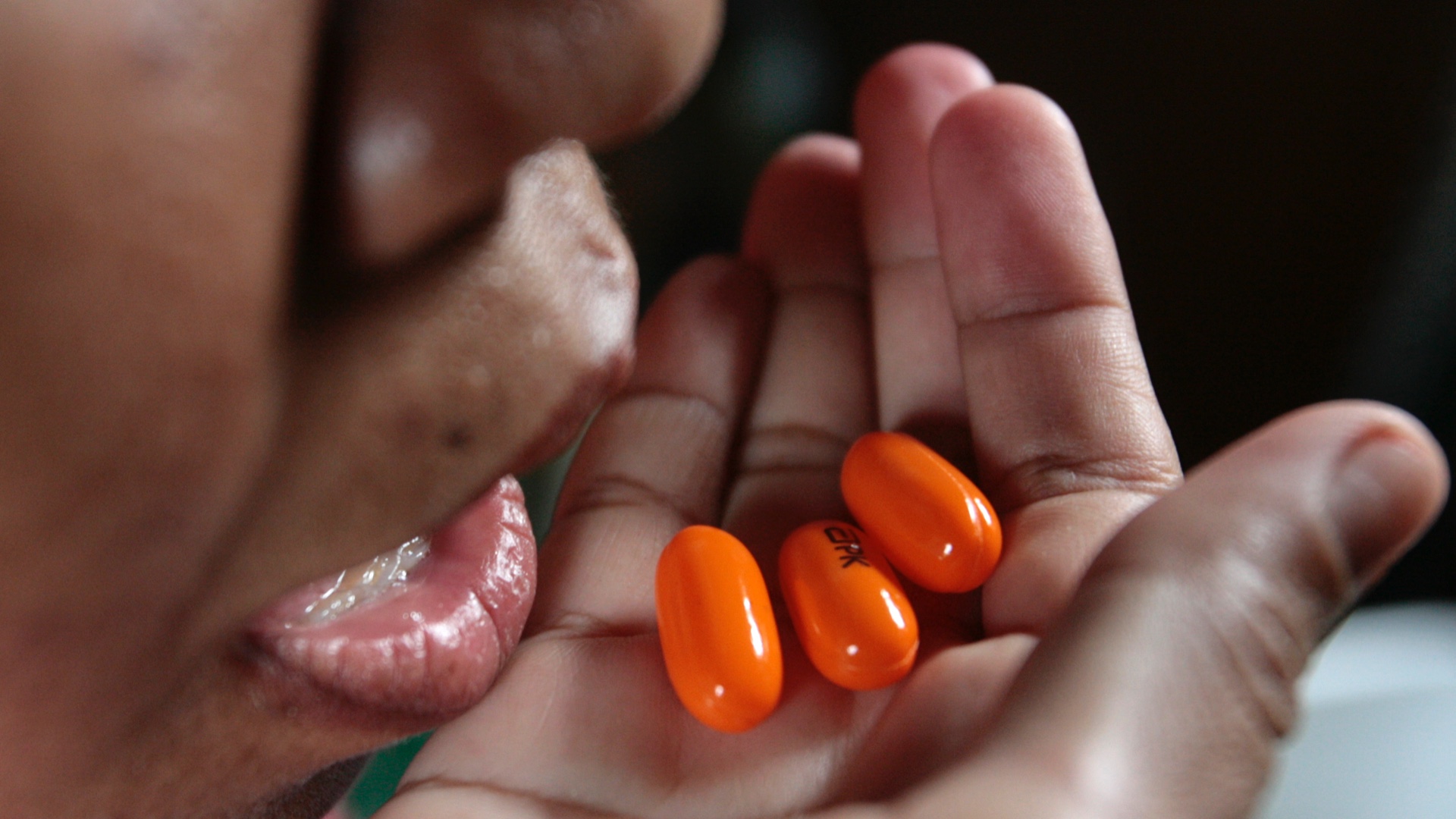
Kaletra
The WHO continues to look at other drugs that may help those suffering from moderate to severe symptoms of COVID-19. One of those being tested is a combination of antiviral drugs usually used to treat people with HIV.
The drugs, lopinavir-ritonavir are known under the brand name Kaletra and have been used in the treatment of HIV since 2000 in the United States. Kaletra cannot cure HIV, but it does slow down the rate at which the virus multiplies, reducing viral numbers, or viral load, in the body as well as the risk of it progressing to AIDS.
HIV has enzymes inside it called proteases, which is something the virus needs in order to reproduce. Both ritonavir and lopinavir are protease inhibitors, meaning they help slow down the reproduction of the virus. Lopinavir does this by blocking the enzyme, while ritonavir stops the liver from breaking down lopinavir so that it stays in the body for longer, thereby working for longer.
As well as being used to treat HIV, Kaletra has also been trialled as a potential treatment for another type of coronavirus – Middle East respiratory syndrome (MERS). In 2015, a Chinese study looked at the drug’s ability to treat MERS in monkeys, with promising results. Although more work is needed, it showed significantly improved survival rates in monkeys infected with the MERS virus who were treated with Kaletra compared with those who were not.
This prompted researchers in Wuhan, China to evaluate the effectiveness of the drug on people with COVID-19. The study, published in The New England Journal of Medicine, looked at 199 patients who were in hospital with COVID-19. It treated 99 of them with Kaletra while 100 were given standard care. The results were not encouraging; they concluded that in hospitalised adult patients with severe COVID-19, there was no benefit from lopinavir-ritonavir treatment.
Clearly, we cannot draw any significant large-scale conclusions from one study, which is why the WHO is looking into this further and on a much larger scale. Another arm of the WHO study is looking at Kaletra being given alongside a molecule known as interferon-beta, which is sometimes used to treat multiple sclerosis and has been shown to regulate inflammation in the body. It was also used alongside HIV drugs to treat monkeys with MERS and showed some promising results.
It will have to be used very carefully in COVID-19 cases, as too little inflammation can result in a weakened immune response to the infection, while too much may lead to an over-response and cause further complications.
It is still early days and recruiting thousands of patients in different countries is a challenge in itself. But robust research on a large scale will be the only way to find a safe and effective treatment for this virus while we wait for an effective vaccine to be produced.