Malaria vaccine a ‘breakthrough for science’, WHO chief says
UN health body announces the Mosquirix vaccine against malaria should be widely administered to children in Africa, a move saving tens of thousands of lives.
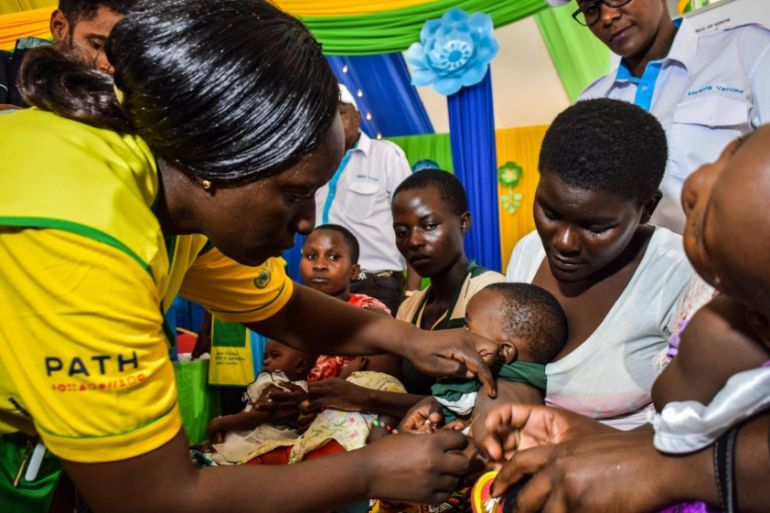
The World Health Organization (WHO) said on Wednesday the only approved vaccine against malaria should be widely given to African children, marking a major advance against a disease that kills hundreds of thousands of people annually.
The WHO recommendation is for RTS,S, sold as “Mosquirix”, a vaccine developed by British drugmaker GlaxoSmithKline.
Keep reading
list of 4 itemsCoronavirus or malaria, tuberculosis and HIV?
India makes ‘impressive gains’ in fight against malaria: WHO
Malaria gains at risk from COVID-19 pandemic: WHO
Since 2019, 2.3 million doses of Mosquirix have been administered to infants in Ghana, Kenya and Malawi in a large-scale pilot programme coordinated by the WHO. The majority of those whom the disease kills are aged under five.
That programme followed a decade of clinical trials in seven African countries.
“This long-awaited malaria vaccine is a breakthrough for science. This is a vaccine developed in Africa by African scientists and we’re very proud,” said WHO Director-General Tedros Adhanom Ghebreyesus.
“Using this vaccine in addition to existing tools to prevent malaria could save tens of thousands of young lives each year,” he added, referring to anti-malaria measures such as bed nets and spraying.
Malaria is far more deadly than COVID-19 in Africa. It killed 386,000 Africans in 2019, according to a WHO estimate, compared with 212,000 confirmed deaths from COVID-19 in the past 18 months.
The WHO says 94 percent of malaria cases and deaths occur in Africa, a continent of 1.3 billion people. The preventable disease is caused by parasites transmitted to people by the bites of infected mosquitoes; symptoms include fever, vomiting and fatigue.
The vaccine’s effectiveness at preventing severe cases of malaria in children is only about 30 percent, but it is the only approved vaccine. The European Union’s drugs regulator approved it in 2015, saying its benefits outweighed the risks.
“This is how we fight malaria, layering imperfect tools on top of each other,” said Ashley Birkett, who leads global malaria vaccine work at Path, a non-profit global health organisation that funded the development of the vaccine with GSK and the three-country pilot.
Another vaccine against malaria, developed by scientists at the UK’s University of Oxford and called R21/Matrix-M, showed up to 77 percent efficacy in a year-long study involving 450 children in Burkina Faso, researchers said in April, but it is still in the trial stages.
GSK also welcomed the WHO recommendation.
“This long-awaited landmark decision can reinvigorate the fight against malaria in the region at a time when progress on malaria control has stalled,” Thomas Breuer, chief global health officer, said in a statement.
Funding challenge
Experts said the challenge now will be mobilising financing for the production and distribution of the vaccine to some of the world’s poorest countries.
GSK has to date committed to producing 15 million doses of Mosquirix annually, in addition to the 10 million doses donated to the WHO pilot programmes, up to 2028 at a cost of production plus no more than 5 percent margin.
A global market study led by the WHO this year projected demand for a malaria vaccine would be 50 to 110 million doses per year by 2030 if it is deployed in areas with moderate to high transmission of the disease.
The GAVI vaccine alliance, a global public-private partnership, will consider in December whether and how to finance the vaccination programme.
“As we’ve seen from the COVID vaccine, where there is political will, there is funding available to ensure that vaccines are scaled to the level they are needed,” said Kate O’Brien, director of WHO’s Department of Immunization, Vaccines and Biologicals.
A source familiar with planning for the vaccine’s development said the price per dose was not yet set, but would be confirmed after GAVI’s funding decision and once there is a clear sense of demand for the vaccine.
Germany’s BioNTech, which developed a coronavirus vaccine with US giant Pfizer, also said it aimed to start trials for a malaria vaccine next year using the same breakthrough mRNA technology.
The WHO also hopes this latest recommendation will encourage scientists to develop more malaria vaccines.
The WHO’s decision had personal meaning for Dr Rose Jalong’o, a vaccinology specialist at the Kenyan health ministry.
“I suffered from malaria as a child and during my internship, and during my clinical years, I attended to children in hospital because of severe malaria who needed a blood transfusion and unfortunately, some of them died.
“It’s a disease I have grown up with and, seeing all this in my lifetime, it’s an exciting time.”