Trump signs off on $8.3bn in spending for coronavirus
Funding will provide government support for state and local public health systems, hospitals and medical centres.

Washington, DC – US President Donald Trump on Friday signed an $8.3bn measure to help tackle the coronavirus outbreak that provides federal public health agencies with money for vaccines, tests and potential treatments and helps state and local governments prepare and respond to the threat.
About $435m of the spending is designated for health systems in other countries, and $300m for humanitarian aid.
Keep reading
list of 3 itemsCoronavirus sparks concerns over European financial operations
Coronavirus: Airlines face losses of more than $100bn
Incidence of the coronavirus is rising in the United States as people showing symptoms of the highly contagious disease COVID-19 seek treatment, are tested and diagnosed.
“The risk in the United States as whole, is still low,” Dr Anthony Fauci, the chief infectious disease specialist at the US National Institutes of Health, told reporters at the White House on Thursday.
“About 80 percent or more of individuals would do well without needing any medical intervention. However, if you are elderly, and have underlying conditions … the risk of those individuals getting into trouble is significantly higher.”
|
|
US cases of coronavirus infection numbered 162 in 15 states as of Thursday, according to researchers at Johns Hopkins University tracking the spread of the virus, but are now changing almost hourly. There have been 11 confirmed deaths from the virus.
“When you take the number of cases we know about, that is a snapshot of what’s there two, three weeks ago,” Ken Cuccinelli, deputy secretary for Homeland Security, told Congress on Thursday.
The Trump administration last week formed a government taskforce under Vice President Mike Pence to address the growing crisis in the US and has been scrambling to respond to the threat of the virus.
“I guess we’ve got to call it a pandemic at this point in time,” said Senator Ron Johnson, chairman of the Senate Homeland Security Committee which held a hearing on the Trump administration’s actions to date.
“There is an awful lot about this disease which nobody knows,” Johnson said. “Hopefully, this will prove to be somewhat seasonal although there are indications it may not be.”
The WHO has not yet declared the global spread of the virus a pandemic, a designation that involves widespread global contagion, but said on Wednesday the rate of death is likely 3.4 percent based on data from China, higher than previously thought.
President Trump on Wednesday night disputed the WHO’s latest assessment of the risks of the virus in a telephone call to a US television talk show. Trump said he had a “hunch” based on conversations that the mortality rate was likely 1 percent.
“Well, I think the 3.4 percent is really a false number,” Trump said.

The Trump administration is beginning to impose travel bans and quarantines to try to contain the entry of people carrying the virus into the US.
People who have been to Hubei province, China, are subject to mandatory quarantine. People travelling from elsewhere in China and Iran are subject to screening and voluntary isolation. This has affected more than 53,000 travellers to date, Cuccinelli.
Separately, the US State Department is implementing “aggressive travel restrictions” Secretary of State Mike Pompeo said at a State Department news conference on Thursday.
“We are working with Italy and South Korea, two countries hit especially hard, to create effective passenger screenings for passengers coming to the United States,” Pompeo said.
South Korea has reported 5,328 cases and Italy 3,089, according to the WHO.
“We have also extended offers to help the Islamic Republic of Iran. And we are hoping the government of Iran will heed our offers of humanitarian assistance and medical supplies,” Pompeo said.
The US coastguard is now reviewing reports of passenger travel and health from inbound cruise ships. Cruise liners are avoiding taking on passengers from hot zones so they do not have to quarantine an entire ship offshore.
A coastguard helicopter dropped coronavirus test kits to a cruise ship bound for San Francisco that is being monitored, according to Cuccinelli.
“We really are handling them one by one, but it is with an eye to not bringing ashore virus spread,” Cuccinelli said.
California Governor Gavin Newsom on Wednesday declared a state of emergency for the entire state with 62 confirmed cases of COVID-19 and one death.
More than 9,400 people in California were being monitored. Florida and Washington state, where 10 people have died, declared a state of emergency over the weekend.
The number of confirmed cases is rising in the US as test kits become available to doctors and hospitals treating patients with symptoms of COVID-19.
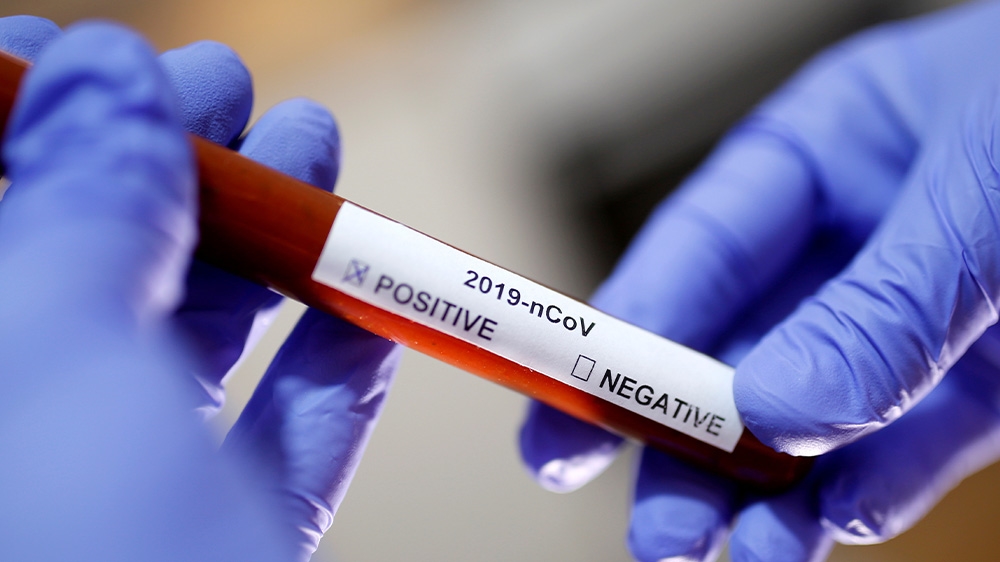
The CDC has distributed 75,000 test kits that detect the presence of the virus to medical labs around the country and more test kits will become available in coming weeks, Dr Robert Kadlec, assistant secretary of Health and Human Services told Congress on Thursday.
Kadlec said 2,500 kits with 500 tests each, manufactured by a private company, Integrated DNA Technologies, are being sent to labs around the country.
The kits will not be immediately available because lab personnel must be trained and validated, but are expected to come into use over the next two weeks, Kadlec said
“That will significantly enhance the ability of doctors and first-line primary care physicians and nurses to judge when their patients should get tested if they suspect coronavirus,” Kadlec said.
A parallel effort by HHS’s Biomedical Advanced Research and Development Authority (BARDA) is under way with other US medical manufacturers to develop more tests, Kadlec said.